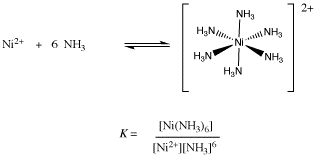Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitr
Draw the structures of [Co(NH3)6]^3+, [Ni(CN)4]^2- and [Ni(CO)4]. - Sarthaks eConnect | Largest Online Education Community
![Explain the type hybridisation, magnetic property and geometry for [ Ni (CN)4 ]^2 - and [ Ni (NH3)4 ]^2 + using VB theory. Explain the type hybridisation, magnetic property and geometry for [ Ni (CN)4 ]^2 - and [ Ni (NH3)4 ]^2 + using VB theory.](https://haygot.s3.amazonaws.com/questions/633645_607318_ans_06ef044c85004ad2aed957fc1fd1f24d.png)
Explain the type hybridisation, magnetic property and geometry for [ Ni (CN)4 ]^2 - and [ Ni (NH3)4 ]^2 + using VB theory.
![Why is [Ni (NH3)6]2+paramagnetic while [Ni (CN) 6]4-diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium Why is [Ni (NH3)6]2+paramagnetic while [Ni (CN) 6]4-diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium](https://miro.medium.com/v2/resize:fit:1182/0*DWO_1XXK-IxUiSTt.jpg)
Why is [Ni (NH3)6]2+paramagnetic while [Ni (CN) 6]4-diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
Draw the structures of [Co(NH3)6]^3+, [Ni(CN)4]^2- and [Ni(CO)4]. - Sarthaks eConnect | Largest Online Education Community
1. Details of Module and its structure Module Detail Subject Name Chemistry Course Name Chemistry 03 (Class XII, Semester 01) Mo
![Under the valence bond approach explain the shape and magnetic behaviour of [Ni(NH3)6]2+.[Given At. No. of Ni = 28] - Wired Faculty Under the valence bond approach explain the shape and magnetic behaviour of [Ni(NH3)6]2+.[Given At. No. of Ni = 28] - Wired Faculty](https://www.wiredfaculty.com/application/zrc/images/qvar/CHEN12070315.png)

![Answered: [Ni (NH3) 6] C12 Explain the… | bartleby Answered: [Ni (NH3) 6] C12 Explain the… | bartleby](https://content.bartleby.com/qna-images/question/33dc3186-d7b9-4388-8560-215f799e8fe0/013f6e76-4df4-4063-bb5c-5eb17a580f72/z92qakc_thumbnail.png)
![Cr(NH3)6]^3 + is paramagnetic while [Ni(CN)4]^2 - is diamagnetic. Explain why? Cr(NH3)6]^3 + is paramagnetic while [Ni(CN)4]^2 - is diamagnetic. Explain why?](https://d1hhj0t1vdqi7c.cloudfront.net/v1/c1hndWJFd3dMMG8=/sd/)

![PDF) Synthesis, structure and thermal decomposition of [Ni(NH3) 6][VO(O2)2( NH3)]2 PDF) Synthesis, structure and thermal decomposition of [Ni(NH3) 6][VO(O2)2( NH3)]2](https://i1.rgstatic.net/publication/256461217_Synthesis_structure_and_thermal_decomposition_of_NiNH3_6VOO22NH32/links/598c15b1458515c333a5e1ff/largepreview.png)
![How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora](https://qph.cf2.quoracdn.net/main-qimg-61bcbbeed69e0a294c52675e216d428f.webp)
![Characteristic bands in infrared spectrum of [Ni(NH3) 6 ][VO(O 2 ) 2... | Download Table Characteristic bands in infrared spectrum of [Ni(NH3) 6 ][VO(O 2 ) 2... | Download Table](https://www.researchgate.net/publication/256461217/figure/tbl1/AS:667594596024340@1536178363556/Characteristic-bands-in-infrared-spectrum-of-NiNH3-6-VOO-2-2-NH-3-2.png)

![How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora](https://qph.cf2.quoracdn.net/main-qimg-f7fd36508df0305723aa4956637d3097.webp)